Miftakhov, Abdusheva, Christensen, 1999
Model Status
This CellML model represents the smooth muscle cell described in the published paper (equations 20-27). The model runs in both OpenCell and COR and the units are consistent. However the model does not replicate the results in figure 2 of the paper - this is most likely to be due to a lack of a stimulus input. The original paper describes several cell types which are linked together, and a mechanical stimulus which is detected by a neuron (eq. 31). Therefore to get this model to run correctly the other cell models will have to be coded up and linked together. CellML 1.1 is suited for this purpose.
Model Structure
ABSTRACT: A complete mathematical model of the periodic myoelectrical activity of a functional unit of the small intestine is presented. Based on real morphological and electrophysiological data, the model assumes that: the functional unit is an electromyogenic syncytium; the kinetics of L-type Ca2+, T-type Ca2+, Ca2+-activated K+, voltage dependent K+and Cl-channels determine the electrical activity of the functional unit; the enteric nervous system is satisfactorily represented by an efferent cholinergic neuron that provides an excitatory input to the functional unit through receptor-linked L-type Ca2+channels and by an afferent pathway composed of the primary and secondary sensory neurons; the dynamics of propagation of the wave of depolarization along the unmyelinated nerve axons satisfy the Hodgkin-Huxley model; the electrical activity of the neural soma reflects the interaction of N-type Ca2+channels, Ca2+-activated K+and voltage dependent Na+, K+and Cl-channels; the smooth muscle syncytium of the locus is a null-dimensional contractile system. With the proposed model the dynamics of active force generation are determined entirely by the concentration of cytosolic calcium. The model describes: the mechanical excitation of the free nerve endings of the mechanoreceptor of the receptive field of the pathway; the electrical processes of the propagation of excitation along the afferent and efferent neural circuits; the chemical mechanisms of nerve-pulse transmission at the synaptic zones; the slow wave and bursting type electrical activity; cytosolic calcium concentration; the dynamics of active force generation. Numerical simulations have shown that the model can display different electrical patterns and mechanical responses of the locus. The results show good qualitative and quantitative agreement with the results of experiments conducted on the small intestine.
The original paper reference is cited below:
Numerical Simulation of Motility Patterns of the Small Bowel. 1. Formulation of a Mathematical Model, R. N. Miftakhov, G. R. Abdusheva and J. Christensen, 1999, Journal of Theoretical Biology, 197, 89-112. PubMed ID: 10036210
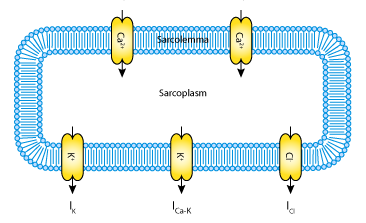 |
| The myoelectrical activity of the gastrointestinal smooth muscle cell is governed by the dynamics of voltage-dependent Ca2+ L and T-type channels (ICa,L and ICa,T), a voltage-gated K+ channel (IK), a calcium-activated K+ channel (ICa-K), and a leak chloride current (ICl). |
