Bertram, Sherman, 2004
Model Status
This model is has consistent units and has been verified as valid CellML by ValidateCellML. It is currently unsuitably constrained and can not be solved.
Model Structure
Pancreatic beta-cells are located in clusters within the pancreas called the islets of Langerhans. Beta-cells secrete the hormone insulin in response to elevated blood glucose levels, and in doing so, they play an essential role in glucose homeostasis. When beta-cells fail to function properly, this can lead to pathologies such as type II diabetes.
Insulin secretion is oscillatory, and it is in-phase with oscillations in the free cytosolic calcium concentration ([Ca2+]i), and theses Ca2+ oscillations reflect a bursting pattern in the beta-cell electrical activity. Electrical bursting consists of periodic active phases of cell firing (excitation) followed by silent phases of hyperpolarisation (rest). These oscillations can be divided into three categories:
-
Fast bursting, which has a period between 2 and 5 seconds and which often occurs in single cells and in islets where acetylcholine is present;
-
Medium bursting, which has a period of 10 to 60 seconds and which occurs in islets where there is a stimulatory glucose concentration; and
-
Slow bursting, which has a period of 2 to 4 minutes and which occurs in single cells and in islets.
The first mathematical models of beta-cells were developed to describe medium bursting, and the first models to address the variability in beta-cell oscillations were developed by Chay in 1995 and 1997 (see Extracellular and Intracellular Calcium Effects on Pancreatic Beta Cells, Chay, 1997 for more details). In these models the main mechanism for oscillations was variation in the Ca2+ concentration in the ER, which directly or indirectly modulates one or more Ca2+-dependent channels. In the Bertram and Sherman model described here the authors analyse in detail how the ER exerts its affects using a phantom bursting model (see the figure below).
The phantom bursting model is a general paradigm for temporal plasticity in bursting in beta-cells in which bursting is driven by the interaction of two slow variables with disparate time constants (see The Phantom Burster Model for Pancreatic Beta-Cells, 2000 for more details). There are three potential slow variables which could drive the phantom bursting in vivo:
-
cytosolic Ca2+ concentration;
-
ER Ca2+ concentration;
-
and the ADP to ATP ratio.
The complete original paper reference is cited below:
A Calcium-based Phantom Bursting Model for Pancreatic Islets, Richard Bertram and Arthur Sherman, 2004, Bulletin of Mathematical Biology , 66, 1313-1344. (Full text (HTML) and PDF versions of the article are available to subscribers on the Bulletin of Mathematical Biology website.) PubMed ID: 15294427
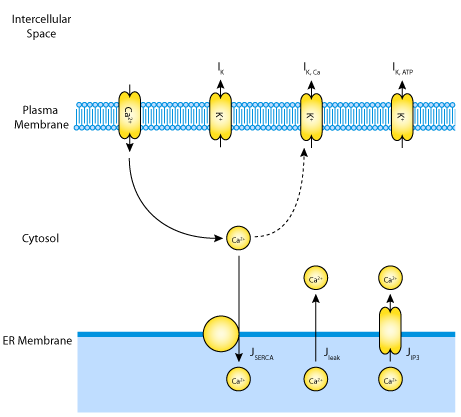 |
| A schematic diagram of the ionic currents and fluxes across the ER and the cell surface membranes, which are described by the mathematical model. |
