Seemann, Sachse, Weib, Dossel, 2003
Model Status
This model contains partial differentials and as such can not currently be solved by existing CellML tools.
Model Structure
During a cardiac cycle, electrical excitation causes a mechanical contraction of the heart. This electromechanical coupling is controlled by free cytosolic calcium. Regional heterogeneity of the electrophysiological properties within the human ventricles is based on changes in ion channel kinetics and density withinh the myocytes of the heart walls. This heterogeneity not only influences the electrophysiological properties of the heart, but also cellular force devlopment.
In the Seemann et al. 2003 publication described here, the authors aim to fit new parameters based on experimental data to an existing mathematical model of a human myocyte, and to investigate the effects of these new values on electrophysiology and force development in a computer model. The cellular model consists of two submodels. One model is needed to describe the electrophysiology of the cell (see the first figure below). This part of the model is based on the previously published Priebe and Beuckelmann, Electrophysiological Model of the Human Ventricular Myocyte, 1998. The second model is needed to quantify the functionality of the contractile units (see the second figure below). This part of the model is based on the publication by Sachse et al.; Modelling the Protein Interactions Involved in Cardiac Tension Development, 2003.
The complete original paper reference is cited below:
Quantitative Reconstruction of Cardiac Electromechanics in Human Myocardium: Regional Heterogeneity, Gunnar Seemann, Frank B. Sachse, Daniel L. Weiss, and Olaf Dossel, 2003, Journal of Cardiovascular Electrophysiology , 14, S219-S228. (Full text (HTML) and PDF versions of the article are available to subscribers on the Journal of Cardiovascular Electrophysiology website.) PubMed ID: 14760927
In order to complete the model, some parameters were taken from the following publication: Quantitative Reconstruction of Cardiac Electromechanics in Human Myocardium: Assembly of Electrophysiologic and Tension Generation Models, Frank B. Sachse, Gunnar Seemann, Kraisorn Chaisaowong, and Daniel Weiss, 2003, Journal of Cardiovascular Electrophysiology , 14, S210-S218. (Full text (HTML) and PDF versions of the article are available to subscribers on the Journal of Cardiovascular Electrophysiology website.) PubMed ID: 14760926
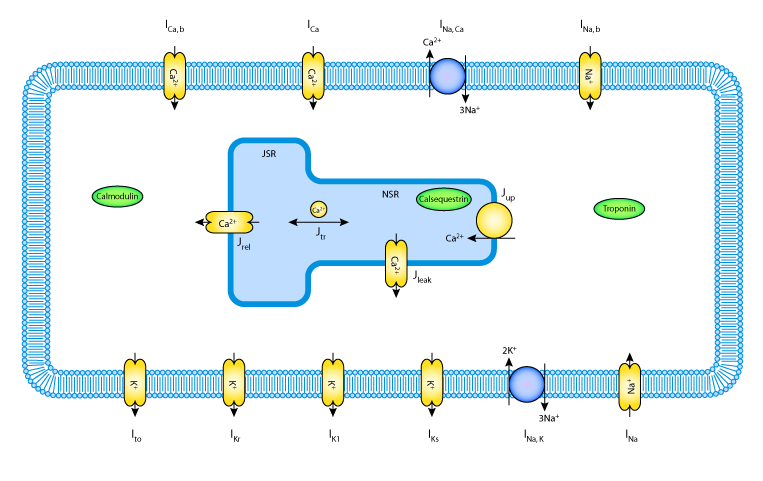 |
| A schematic diagram describing the transmembrane ionic currents through channels, pumps and exchangers, that are captured in the Priebe and Beuckelmann 1998 model of a human ventricular myocyte. The intracellular compartment in the diagram represents the sarcoplasmic reticulum (SR). This is divided into the two subcompartments, the network SR (NSR) and the junctional SR (JSR). Ca2+ buffers are present in both the cytoplasm and the JSR - troponin (TRPN), Calmodulin (CMDN), and calsequestrin (CSQN). |
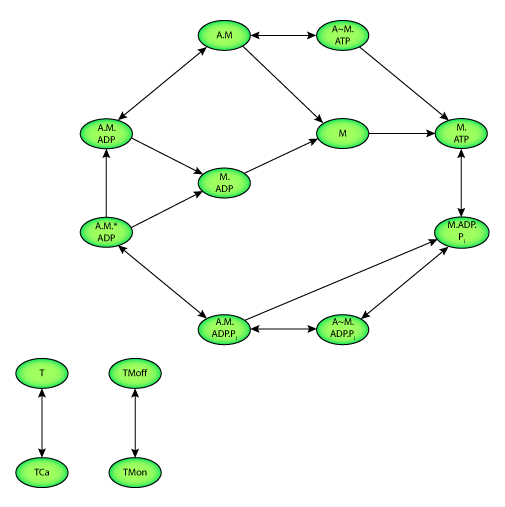 |
| State diagram of the Sachse et al. 2003 model. Two state variables quantify the calcium binding to troponin C (T). Two further state variables describe the configuration of tropomyosin (TM). Ten state variables represent the interaction of actin and myosin as well as the hydrolysis of adenosine triphosphate (ATP). M and A represent myosin and actin respectively. Transition between states is depicted by an arrow. |
