Modeling the Recruitment and Synchronization of SMCs (Koenigsberger et al. 2004)
About this model
| Original publication: | |
|---|---|
| Koenigsberger, Michèle, et al. (2004): "Ca2+ dynamics in a population of smooth muscle cells: modeling the recruitment and synchronization." Biophysical journal 87.1 (2004): 92-104. | |
| DOI: | 10.1529/biophysj.103.037853 |
Model status
The current CellML implementation runs in OpenCOR. The results have been validated against the data extracted from the figures in the published Koenigsberger, Michèle, et al. (2004). We provide the settings used for the figure reproduction with the simulation results shown under Experiments. The model structure can be found in the documentation of Components. The curation process has been summarized in the Model history and Known issues.
Model overview
The Koenigsberger, Michèle, et al. (2004) proposed a model describing a population of coupled smooth muscle cells (SMCs), while the calcium dynamics in a single SMC extended the model of Parthimos et al.(1999). This workspace holds a CellML encoding of the Koenigsberger, Michèle, et al. (2004) single SMC model.
Modular description
Components
CellML divides the mathematical model into distinct components, which are able to be re-used. Since we have implemented the Gosak, Marko, et al (2014) model, which was also based on the mathematical formulation of the Koenigsberger, Michèle, et al. (2004) model, we reuse some existing components defined in the workspace Single PASMC model (Gosak et al 2014). However, the definition of some flux directions are different, we modify the signs in corresponding terms to reflect it.
The main CellML components are:
- The major elements involved in transplasmalemmal ion exchange, including:
- The calcium influx through Voltage-operated Ca2 + channels (VOCC): reuse the component JVOCCi that has an opposite direction.
- The calcium extrusion by Ca2 + -ATPase pumps, Jextrusion : reuse the component JPMCAi that has the same definition.
- The Na + ⁄ Ca2 + exchange JNa ⁄ Cai : reuse the component JNCXi that has an opposite direction.
- The flux through Na + ⁄ K + ATPase : the JNKAi definition in the Gosak, Marko, et al (2014) model is different from the Koenigsberger, Michèle, et al. (2004) model, hence this component is newly defined in new JNKAi.
- The K + efflux through the voltage-operated K + channels (KC): reuse the component JKi that has the same definition.
- The flux via the Cl − channels: while the JCli in the Gosak, Marko, et al (2014) model is the same but with a constant reversal potential vCl, a new JCli is defined to incorporate the Nernst equation for vCl.
- Intracellular Ca2+ handling, including:
- The SR uptake JSRuptakei: reuse the component JSERCAi that has the same definition.
- Calcium Induced Calcium Release (CICR) via the ryanodine receptors: reuse the component JCICRi that has the same definition.
- Basal Ca2+ leak: reuse the component Jleaki that has the same definition.
- The flux related to the IP3 concentration: a new component defined in JIP3i .
- The flux related to the PLC − δ: a new component defined in JPLCdeltai .
- The flux due to IP3 degradation: a new component defined in Jdegradi .
Ca2 + concentration in the cytosol (c): since the Cai definition in the Gosak, Marko, et al (2014) model does not incorporate JIP3i, this component in the Koenigsberger, Michèle, et al. (2004) model is newly defined in new Cai, which also reflects the differences of the fluxes direction definition mentioned above.
Ca2 + concentration in sarcoplasmic reticulum (s): reuse the component Casr that has the same definition.
The dynamics of the cell membrane potential (v): reuse the component Vm that has the same definition, while the differences of the fluxes direction definition mentioned above are already incorporated.
IP3 concentration in the cell (I): a new component defined in IP3
Each of these blocks is itself a CellML model, which enables us to reuse the various components in future studies and models.
Experiments
Following best practices, this model separates the mathematics from the parameterisation of the model. The mathematical model is imported into a specific parameterised instance in order to perform numerical simulations. The default parameters are defined in Para. The parameterisation would include defining the stimulus protocol to be applied.
This workspace encodes Single-cell response experiment and corresponding simulation results.
Simulation settings
Simulation settings are encoded in SED-ML files for experiment execution. It is common that we may need to vary experimental settings to obtain data under various conditions. Hence, the full experimental settings are encoded in the simulation scripts. The Python scripts to run simulation and reproduce the figures in the original paper are included under the Simulation/src folder. The runSim.ps1 and runSim_7.ps1 is used to run the simulation in PowerShell, while plotFig2_1.py, plotFig2_2.py, plotFig3.py , plotFig7_1.py, plotFig7_2.py, plotFig7E_1.py and plotFig7E_2.py are ready to plot the figures in the Single-cell response experiment.
Model history
There is no publicly available code for this model.
Known issues
- The parameters and initial values listed in Table 1 are not explicitly present in the primary paper. While the model curator used the values from the cited references or assumed some parameters based on the text description, these values may not be the ones that the primary publication used.
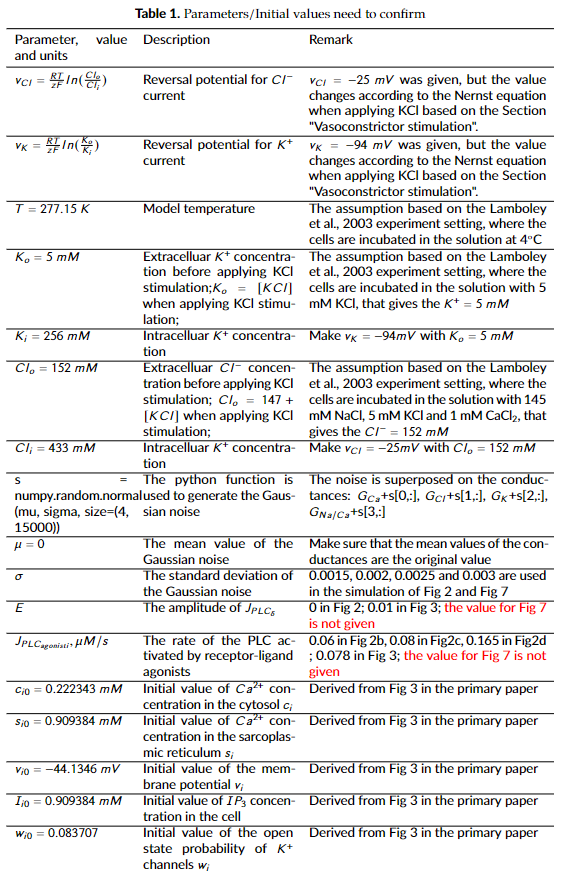
- Fig 3 matches the original data well, while the simulation results of Fig 2 and 7 are not exactly align with the original data, which could be caused by the parameter settings in Table 1 and the stochastic nature of the simulation settings.
